Abstract
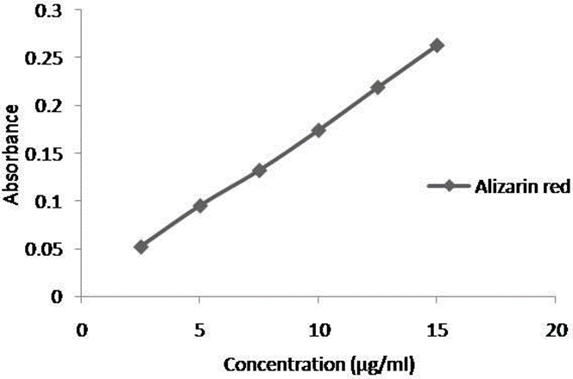
Simple, sensitive and rapid extractive spectrophotometric method was developed for the determination of solifenacin in bulk and pharmaceutical dosage form. This method was based on the formation of yellow ion-pair complex between the basic nitrogen of the solifenacin and alizarin red in acid solution. The formed complex was extracted with chloroform and the absorbance was measured at 430 nm. The system obeyed Beer’s law in the ranges 2.5–22.5 µgmL−1. The developed method was validated as per ICH guidelines. The effect of optimum conditions via strength of the acid solution on the ion pair formation, reagent concentration, time and temperature, and solvent was studied. The low relative standard deviation (%RSD) values indicate good precision and high recovery values. This method has been successfully applied for the assay of solifenacin in pure form and in pharmaceutical formulations.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.