Abstract
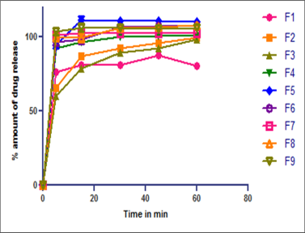
Orodispersible Tablets (ODT) is a novel tableting technology which is formulated, and it overcomes the difficulties of other multi compressed tablets. Telmisartan has a bioavailability of 42-100 percent and a 24-hour elimination half-life. It excretes the majority of drugs through the faeces, which accounts for 97 percent of total drug excretion. The objective of this research is to formulate and evaluate Telmisartan loaded ODT and to prove the enhancement of dissolution and bioavailability of Telmisartan. From the DSC studies, it was confirmed that Telmisartan and excipients used in the formulation are compatible to each other and suitable for the manufacturing process. Telmisartan loaded ODT was formulated by wet granulation technique and evaluated for powder characteristics and release characteristics. About 9 formulations were formulated in each ODT, and all the formulation obeys a good powder flow characteristic from the angle of repose, Carr’s index and Hausner’s ratio. All the experimental formulation batches have been subjected to various evaluations viz, average weight, friability, disintegration, thickness, hardness, dissolution, content uniformity. Among this nine Telmisartan ODT formulations (F1-F9), F7 possess an expected release pattern and disintegration time in a short time period (i.e., 101.8 ± 2.72 in 5th min and disintegration time at 5 seconds), which may fastens the absorption and bioavailability of Telmisartan. It was concluded that ODT was a suitable dosage form to enhance the solubility at the same time the bioavailability of BCS class II drugs like Telmisartan.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.