Abstract
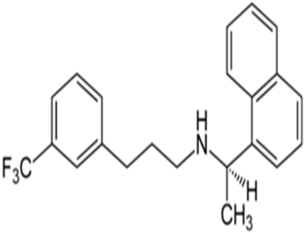
Cinacalcet (INN), a calcium mimetic drug that mimics the activity of calcium in the body. It expresses itself by activation of calcium-sensing receptor allosterically in different organs and tissues. The secretion of parathyroid hormone is regulated principally by calcium-sensing receptors that are present on the surface of the parathyroid gland. Cinacalcet is used in the treatment of hyperparathyroidism, which is the usual consequence of parathyroid cancers and CKF. There had been a rapid increase in the number of drugs that are adding to each class of drugs. These drugs are formulated into newer formulations either in a single or multi-drug dosage forms. These newer marketed formulations demand a new investigation for the estimation of the drug in the formulations. The existing analytical techniques for those drugs are available in the research literature, but not all the methods are stable and economical to use. The objective of this work was to develop an analytical RP-HPLC method for the estimation of cinacalcet in bulk and tablet formulation. The emphasis was given to the short time of analysis, and simplicity in the method. RP-HPLC analysis of the drug satisfies the peak integrity, suitability, recovery of the drug. LOQ and LOD of the drug were achieved with high sensitivity. The data shows the precision of the method and the accuracy of the method. Overall, the data suggest that the proposed analytical method can be used to analyse the drug in the formulation. This method can be recommended for the routine analysis of the drug in its dosage form.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.