Abstract
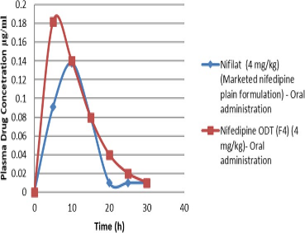
Nifedipine has a bioavailability of 45-56 percent and a 2-hour elimination half-life. It has a 50 percent kidney excretion rate and a 5-15 percent bile excretion rate. The intention of this research is to invent and evaluate Nifedipine loaded ODT and to prove the enhancement of bioavailability. The 23 factorial optimization design exposed about the outcome of independent variable on dependent variable throughout the formulation of Nifedipine ODT. From the records, it was accomplished that there was a good correlation between Disintegration time, Dissolution rate and super disintegration concentration. The formulation F4 (Nifedipine ODT) has achieve the goal of ODT drug delivery with desired release characteristics, cost-effective, decreased dose, effective administration and hence improved patient compliance. The invivo pharmacokinetic studies reveals that increase in AUC0-∞; decrease in Tmax; increase in Cmax in Nifedipine ODT shows better bioavailability and faster duration of therapeutic action than marketed Nifilat® dosage form. Nifedipine ODT was stable at various temperature, humidity conditions and there was no drastic change in evaluation parameters. That it was concluded that Oral dispersible tablet (ODT) was a suitable dosage form to enhance the solubility at the same time the bioavailability of BCS class II drugs like Nifedipine.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.