Abstract
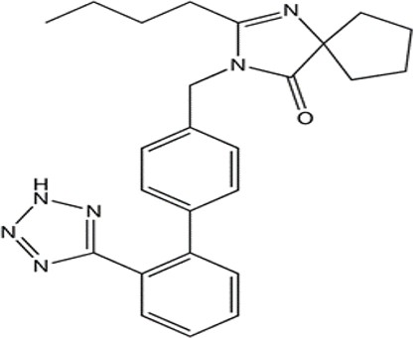
The present study was aimed to develop and validate a simple, sensitive and economical bio-analytical high-performance liquid chromatographicultraviolet method for the determination of irbesartan in human plasma. The method involves the use of simple precipitation method for the determination of irbesartan, using methanol as precipitating agent and losartan as internal standard. The separation was achieved using Zorbax C18 column (150 x 4.6 mm, 5µm), mobile phase consists of methanol and 0.2% formic acid in water at the ratio 85:15, v/v using detection wavelength of 237 nm. Further, the developed method was validated as per US-FDA guidelines for accuracy, precision, linearity, stability, detection and quantification limit. The method developed was found to be linear over the concentration ranging from 5 to 500 ng/ml with a correlation coefficient of 0.9987. The LOD and LLOQ of the method were found to be 1 ng/ml and 5 ng/ml, respectively.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.