Abstract
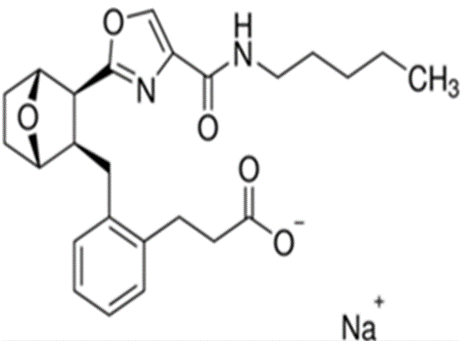
The purpose of this work is to develop and validate reverse phase Ultra performance liquid chromatography (UPLC) method for the rapid and precise determination of ifetroban sodium in its pure form and in formulations. A simple, specific, accurate, precise isocratic UPLC method for analysis of Ifetroban sodium was developed and validated using a Phenomenex C18 column (50 mm x 3.0 mm, 3µ) as the stationary phase, in conjunction using Triethyl amine buffer: methanol in the proportion of 25:75 with a flow rate of 1.0 mL/min, run time is 3 min and UV detector is used at 235 nm wavelength. The developed UPLC technique was found to be rapid as the retention time was 0.56 minutes for Ifetroban peak to elute. The developed UPLC technique was validated as per the ICH guidelines for specificity, linearity, accuracy, precision, robustness and found to be satisfactory. Linearity was established in the concentration range 100-300 µg/mL with correlation coefficient of 0.998 and the equation obtained is y = 0.635x + 0.639. The percentage recovery is 100.41. The method is rugged and is trouble free and transferable. The study showed that the developed UPLC technique can be used for the estimation of drug purity, stability, solubility and with no interference of pharmaceutical excipients from the active pharmaceutical ingredient. The precision, accuracy, robustness results obtained enables rapid quantification of ifetroban for quantitative analysis.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.