Abstract
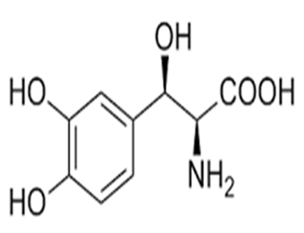
The purpose of this work is to develop and validate stability, indicating reverse phase High-performance liquid chromatography (HPLC) method for the rapid and precise determination of droxidopa in its pure form and formulations. A simple, fast, accurate and economical way has been developed and validated for the quantification of droxidopa by HPLC technique. The chromato graphic system was equipped with Shimpack column C18 ((250x 4.6) mm, 5µ) as stationary phase and UV detector at 220 nm, in conjunction with a mobile phase of phosphate buffer pH 2.0: acetonitrile (60:40,% v/v) at a flow rate of 1.0 mL/min. The developed HPLC technique was found to be rapid as the retention time was 2.2 minutes for droxidopa peak to elute. The method was validated as per the International Conference on Harmonization (ICH) guidelines for specificity linearity, accuracy, precision. The developed method was selective with well-resolved peak. Linearity was observed over the concentration range of 25-150µg/mL for droxidopa. The recovery of Droxidopa was found to be 100.54% - 101.65%. Statistical techniques were employed for the validation of precision, linearity, accuracy, robustness and ruggedness and can be applied for routine analysis. Validation revealed that the developed method was specific, accurate, precise, reliable, robust, reproducible and suitable for the systematic quantitative review.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.