Abstract
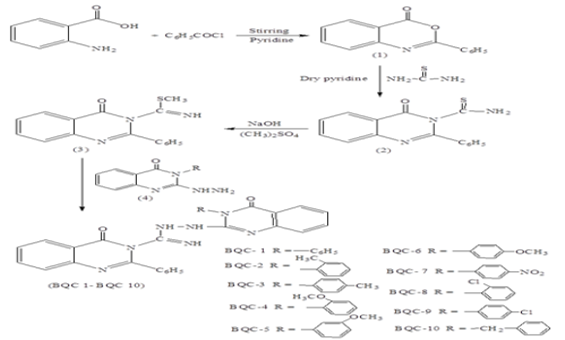
A series of novel 4-oxo-N-(4-oxo-3-substituted-3,4-dihydroquinazolin-2-yl amino)-2-phenylquinazoline-3(4H)-carboxamidines are prepared from methyl 4-oxo-2-phenylquinazoline-3(4H)-carbthioimidate & 3-(substituted)-2-hydrazino-quinazoline-4(3H)-ones. The starting material 4-oxo-2-phenylquinazoline-3(4H)-carbthioimidates were prepared from anthranilic acid while the 3-(substituted)-2-hydrazino-quinazolin-4(3H)-one was prepared from a range of 1° amines using multistep preparation. Entire synthesized analogues were screened for their antitubercular, anti-HIV and antibacterial activity. Among the series, N-(3-(4-nitrophenyl)-4-oxo-3,4-dihydroquinazolin-2-yl amino)-4-oxo-2-phenylquinazoline-3(4H)-carboxamidine (BQC7) and N-(3-(4-chlorophenyl)-4-oxo-3,4-dihydroquinazolin-2-yl amino)-4-oxo-2 phenylquinazoline-3(4H)-carboxamidine (BQC9) showed most potent activity against S. epidermidis, S. aureus & B. subtilis with the MIC of 3 µg/mL. The compound BQC7 displayed the antitubercular potency at 12.5 µg/mL and anti-HIV activity at 8.53 µg/mL against HIV1 and HIV2. Thus, these derivatives are useful in the development of novel antitubercular & antiHIV agents. The results obtained from this study confirm that the synthesized and biologically evaluated quinazolines showed promising antimicrobial, antitubercular and anti-HIV activities and are new scaffolds for antimicrobial activity.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.