Abstract
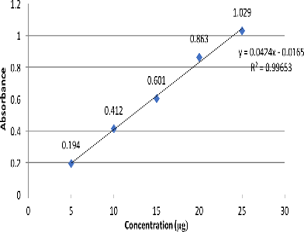
A very simple, non-destructive, inexpensive and green strategy applied for the quantification of Naproxen using transmission Fourier Transform Infrared (FTIR) spectroscopy in bulk & tablet dosage form and the Beer’s concentration range found to be 5-25μg. For the present investigation of Naproxen, O-H, 3000 - 3500cm-1 was selected for the analysis. The correlation coefficient for the method found to be 0.9965 and the developed method analyzed for specificity, limit of detection (LOD), limit of quantification (LOQ), linearity of response, precision and accuracy. This work clearly shows the capability of transmission FTIR spectroscopy for assessment of exact quantity of API to control the quality of finished products as well as during processing in pharmaceutical industries. Therefore, as compared to other spectroscopic or chromatographic method, costly chemicals and toxic solvents totally avoided in this direct, inexpensive and green approach. Such types of FTIR applications have a strong potential to replace classical methods in quality assurance/quality control (QA/QC) for the analysis of active contents in pharmaceuti- cal preparations.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.