Abstract
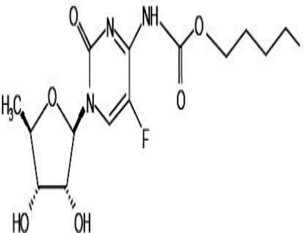
The combination of Irinotecan Hydrochloride (IRI) and Capecitabine (CAP) is indicated for the treatment of cancer. IRI and CAP were developed by an simultaneous simple reverse phase liquid chromatographic method and were subsequently validated from their APIs. The proposed method was based upon the separation of these two chemical agents using Agilent 1200 series HPLC with Qualisil gold C18 (250 × 4.6 mm, 5µ) column and it was maintained at ambient temperature. The effective chromatogram was obtained using the mobile phase of methanol: water (60:40 (% v/v)) and the pH was adjusted to 3 with orthophosphoric acid at the flow rate of 1.0 ml/min. The column effluents were detected using Photo Diode array detector at wavelength of 340 nm. The proposed reverse phase liquid chromatographic method was validated as per ICH Q2 (R1) guidelines. Based upon the optimized parameters, these drugs were effectively separated and the retention time was found at 4.08 min. for IRI and 7.8 min. for CAP with resolution of 7.6. There is no interference with the impurities or degradation products. The calibration plots were found to be linear over the concentration range of 4-24 µg/ml and 40-240 µg/ml respectively. The LOD and LOQ of IRI were found to be 0.12 µg/ml and 0.373 µg/ml while LOD and LOQ of CAP were found to be 0.254 µg/ml and 0.771 µg/ml respectively. The mean percent recovery of triplicate analysis of IRI and CAP were found to be 100.58% and 100.03% respectively. In conclusion, the developed method can be used in the quality control laboratories for the determination of IRI and CAP in APIs.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.