Abstract
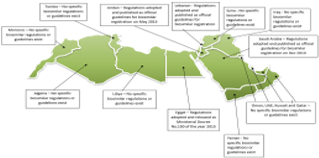
This review article explores the regulatory situation of biosimilar registration pathways in the Middle East and North Africa (MENA). With most countries in the world have either adopted official regulatory guidelines for biosimilar approval or are in the process of developing such guidelines, countries in the MENA region are advised to accelerate the process of adopting pathways for biosimilar approval primarily for preventing the entry of intended copies into such markets and risking patients’ safety in addition to jeopardizing clinical outcomes of the different disease modalities that are treated by biologics. Additionally, biosimilars are playing a significant role in reducing the significant public expenditure on biological therapy and thus increasing the accessibility of these medications to a larger population of patients. The article details the countries in the MENA region that have adopted official and scientific guidelines for biosimilar approval pathways. The article also draws a comparison between different countries on issues such as comparability studies, extrapolation of indications, interchangeability and non-clinical quality requirements. In conclusion, only four countries out of the 15 countries they comprise the MENA region have adopted clear regulatory pathways for biosimilar registration and approval. This situation should be of urgent importance to policymakers responsible for public health bodies in countries that lack such guidelines due to the negative consequences that could result due to the absence of clear biosimilar regulations.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.