Abstract
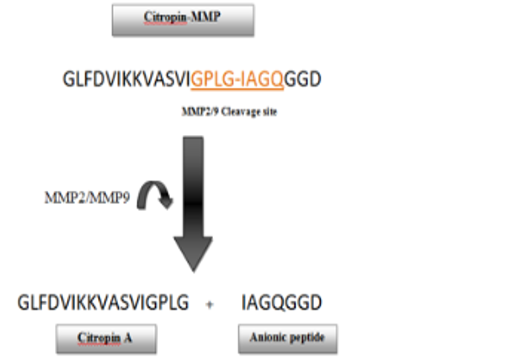
Citropin 1.1 is an amphipathic alpha-helical cationic peptide that exhibits potent anticancer activity in vitro. Citropin 1.1 was found to be active against 60 cancer cell lines, and this activity was mainly attributed to its ability to bind and lyse membranes of cancer cells. One of the major drawbacks of developing Citropin 1.1 as an anticancer agent is its lack of apparent selectivity toward cancer cells and its ability to cause significant lysis of normal human erythrocytes and mammalian cells at high concentrations. This low selectivity index places severe restraints on the development of Citropin 1.1 as a novel anticancer agent. In this study, we have designed a Citropin 1.1 analog named Citropin A that retained the biological activity of the parent peptide. Citropin A was fused to an anionic fragment in order to neutralize the positive charge carried on the parent peptide rendering it inactive. The resultant hybrid peptide named Citropin-MMP was designed to contain a Matrix metalloproteinase (MMP) cleavable consensus sequence that would be cleaved to release the active Citropin A once it encounters highly metastatic MMP producing cancer cells. Citropin-MMP was found to be completely inactive against non-MMP producing cancer cells and normal mammalian cells. However, when Citropin-MMP was administered to MMP producing cells, its antiproliferative activity was regained, and the peptide displayed exclusive activity against MMP producing cancer cell lines. The data of our study indicate that this enzyme-based cleavage strategy could prove to be successful for the development of Citropin-MMP as a novel therapeutic agent for the purpose of inhibiting the proliferation and invasion of highly metastatic invasive cancer cells.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.