Abstract
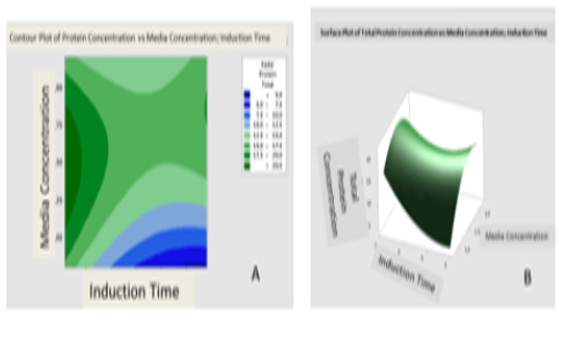
Human Epidermal Growth Factor is techno-economically protein that has the function for stimulates a process of proliferation and cell differentiation. Because of that function, it can be a candidate for wound healing in ulcer diabetic. In the previous study, hEGF can extracellular secreted by OmpA signal peptide using recombinant DNA. The production method of recombinant hEGF will be extracellular secreted by Escherichia coli. However, the optimum secretion of Outer Membrane Protein A (OmpA) has not been studied. Therefore the purpose of this study is to determine the optimum conditions using the RSM method. This research begins with a rejuvenation of culture, extracellular secretion, and optimization with the RSM method, followed by protein production at optimum condition. Secreted recombinant protein measured by Sandwich ELISA method and LOWRY. The result showed that the optimum condition was found in the medium concentration of 1.5x and induction time at 2 hours 10 minutes and the total protein yield of expression was 21.247 mg/mL with optimization percentage 2.96% and efficiency 50.56 % and recombinant protein hEGF concentration in fermentation media 416 μg/mL.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.