Abstract
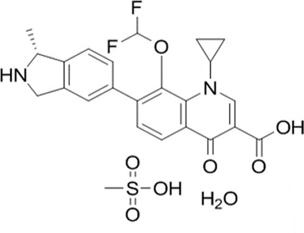
A simple, precise, accurate Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) method was developed for the estimation of Garenoxacin mesylate in human plasma using Ciprofloxacin Hydrochloride as an internal standard. Chromatographic conditions used are stationary phase Zorbax Eclipse XDB C18 (250x4.6 mm, 5m), Mobile phase 0.1% orthophosphoric acid and Acetonitrile in the ratio of 50:50 (% v/v) and flow rate was maintained at 1.0 ml/min, detection wavelength was 240 nm, injection volume of 50 µL and the column temperature was set to 30°C. The retention time of Garenoxacin mesylate was found to be 4.0 min. % Coefficient of Variation of the Garenoxacin mesylate was found to be 4.30. % Recovery was obtained as 98.97%. The linearity of the proposed method was established in the concentration range of 0.04 to 4 µg/ml (Correlation Coefficient = 0.999). The lower limit of quantification was 0.04 μg/ml (S/N Ratio 21) which reach the level drug possibly found in human plasma. Further, the reported method was validated as per the ICH guidelines and found to be well within the acceptable range.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.