Abstract
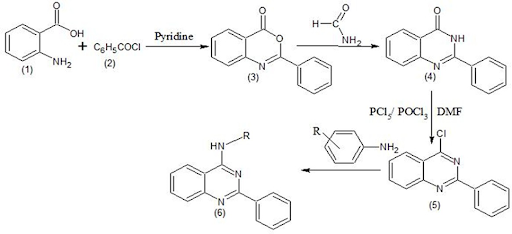
Inh A, the Enoyl Acyl Carrier protein Reductase from Mycobacterium tuberculosis is one of the pivotal enzyme involved in the mycobacterial fatty acid elongation cycle and has been considered as an important target for anti-tubercular screening. Inhibition of Inh A affects the biosynthesis of the mycolic acids, which are the central constituents of the mycobacterial cell wall. In the present research work, 4-anilino quinazoline derivatives were designed based on the quinazoline based drugs by means of lipophilic insertion and Fragment replacement. The designed compounds were synthesized, and molecular docking studies were performed on the human pathogenic bacterial enzyme InhA from its parent domain Mycobacterium tuberculosis. Molecular docking study revealed that compounds SMOQ2, SNAQ3, 4AAQ7, 2AP9, PABAQ10 were found to possess good binding affinity towards the target InhA. With reference to the binding energy obtained from molecular docking study, five compounds were subjected to in vitro anti-tubercular activity against M. tuberculosis H37Rv and I2487 (Resistant strain) using BACTEC MGIT method. Compound SMOQ2 and 4AAQ7 showed sensitivity in both H37Rv (Sensitive strain) and I2487 (Resistant strain) at the concentration of 250, 500, 1000 and 1500 mcg/mL. In silico Pharmacokinetic predictions of the synthesized compounds were determined using SwissADME online web tool. All the synthesized compounds obeyed the Lipinski's rule of five properties.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.