Abstract
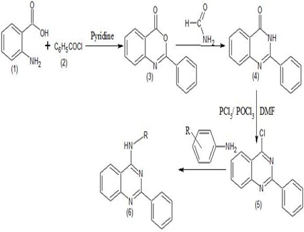
A novel scaffold of 4-anilino quinazoline derivatives was designed on the basis of known inhibitor of quinazoline based drugs. The designed derivatives were synthesized using optimized reaction condition. Their structures were confirmed by FT-IR, 1H-NMR, 13C-NMR and Mass spectral data. The structures of synthesized compounds were subjected to in silico molecular docking using AutoDock software against the target Poly (ADP-ribose) polymerase-1 (PARP-1) enzyme. The compounds were evaluated for their in vitro cytotoxic activity against Daltons Lymphocyte Ascites (DLA) Cell lines. Molecular docking study of the newly synthesized compounds showed good binding mode in the active site of PARP-1. The docking results were compared with the standard drug Doxorubicin. Doxorubicin showed binding energy of -8.94 kcal/mol and formed one hydrogen bond with Asn767 with a distance of 1.98 Å. Compound SMOQ2 showed the least binding energy, i.e., 11.87kcal/mol and formed one hydrogen bond with Arg 878 with a distance of 1.895.A° Compound DMUQ5 showed binding energy of -11.42 kcal/mol and produced two hydrogen bonds with Arg 878 and Asn 767. Among the synthesized compounds, compounds SMOQ2 and DMUQ5 showed significant binding affinity compared to the standard drug Doxorubicin. The in vitro cytotoxic evaluation indicated that compounds SMOQ2 and DMUQ5 showed significant cytotoxic activity against Daltons Lymphoma Ascites cell line.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.