Abstract
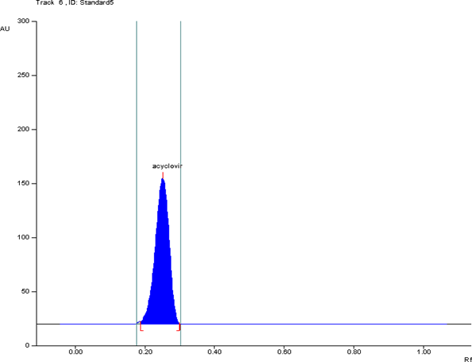
A new, simple, and rapid high-performance thin-layer chromatographic method was developed and validated for quantitative determination of Acyclovir. Acyclovir was chromatographed on silica gel 60 F254 TLC plate using chloroform: methanol: formic acid (6.5 + 3.5 + 0.1 v/v/v) as mobile phase. Acyclovir was quantified by densitometric analysis at 259 nm. The method was found to give compact spots for the drug (Rf = 0.25±0.01). The linear regression analysis data for the calibration plots showed good linear relationship with r2 = 0.9996 in the concentration range 100–600 ng/spot. The method was validated for precision, recovery, repeatability, and robustness as per the International Conference on Harmonization guidelines. The minimum detectable amount was found to be 100 ng/spot, whereas the limit of quantitation was found to be 300 ng/spot. Statistical analysis of the data showed that the method is precise, accurate, reproducible, and selective for the routine quality control of Acyclovir.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.