Abstract
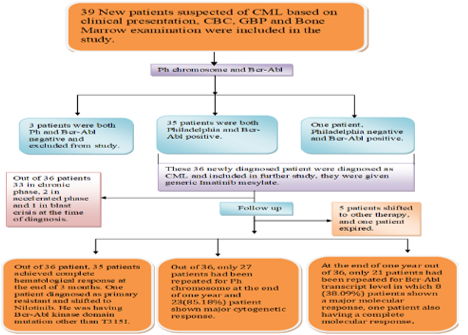
Imatinib is now used as the first-line drug to treat CML patient. However, the emergence of resistance to Imatinib in CML patient, the side effect of bone marrow suppression, fluid overload and gastritis are a major limitation of the use of Imatinib in the treatment of CML. This study was conducted to see the therapeutic response and side effect profile of generic Imatinib Mesylate in newly diagnosed CML patients. All cases of CML were given generic Imatinib and followed prospectively with a minimum follow-up of 6 months. They were followed at an interval of 2 weeks till complete hematologic response, thereafter at an interval of 6 to 8 weeks. Cytogenetic and molecular response at the end of one year also evaluated. Among 36 CML patients, 33 were in chronic phase 2 in accelerated phase and 1 in blast crisis while 35 were Philadelphia+ve and 1 was ph–ve at initial presentation. Minimum duration to achieve CHR was 2 weeks with a mean of 5 weeks. At 3 month except one 35 patients achieved CHR (97%). Out of 36 patients, 27 were subjected for Philadelphia chromosome at one year which shown 23 patients (85.18%) achieved a major cytogenetic response. 8 (38%) patients achieved a major molecular response and one patient (4.76%) was having a complete molecular response at one year. 8 (22.22%) patients developed hematological toxicity to Imatinib with Pancytopenia most common. In conclusion, Generic Imatinib is having an excellent therapeutic response in CML patients although higher response rate may be due to smaller sample size and lesser duration of follow up.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.