Abstract
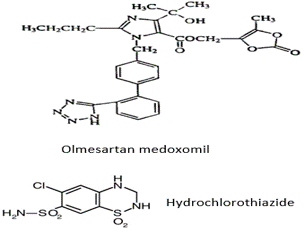
A simple, reproducible and efficient reverse phase high performance liquid chromatographic method was developed for simultaneous estimation of olmesartan medoxomil (OLM) and hydrochlorothiazide (HCTZ) in combined tablet dosage form. Formulation containing OLM with HCTZ are used as antihypertensive angiotensin II receptor blocker. Chromatography was performed on a 250 mm x 4.6 mm, 5-µm particle size, C8 Qualisil BDS column with a 50:50 (v/v) mixture of buffer and acetonitrile as a mobile phase and the pH was adjusted to 4.7 by adding dilute phosphoric acid. The detection of the combined dosage form was carried out at 225 nm and a flow rate employed was 1 ml min-1. The retention times were 5.074 & 7.242 min for olmesartan medoxomil and hydrochlorothiazide, respectively. Linearity was obtained in the concentration range 20 to 100 µg mL-1 for olmesartan medoxomil and in the range 12.5 to 62.5 µg mL-1 for hydrochlorothiazide, with a correlation coefficient of 0.9956 and 0.989. The result of the analysis were validated statistically and recovery studies confirmed the accuracy and precision of the proposed method.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.