Abstract
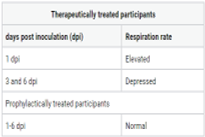
In December 2019, many pneumonia cases were reported without an apparent cause but were associated with seafood and wet markets in Wuhan, China Clinical features were similar to pneumonia, and severe acute respiratory syndrome (SARS-CoV-2) (formerly 2019-nCoV) was determined as the causative pathogen. To date, there are no effective antiviral drugs for the targeted treatment of coronavirus disease 2019 (COVID-19). We provide the VITYALA YETHINDRA (VYTR) hypothesis for the prophylactic and therapeutic efficacy of GS-5734 treatment in humans with COVID-19. Prophylactic GS-5734 treatment may prevent SARS-CoV-2 induced disease and lung lesions in participants inoculated with SARS-CoV-2 and potentially inhibit SARS-CoV-2 replication. Therapeutic GS-5734 treatment may show a reduction in the severity of symptoms, reduce viral replication, the absence of lung lesions in some participants and decrease in lesions in 50% of participants may be possible. As broad-spectrum drugs are capable of inhibiting coronavirus infections, GS-5734 should be considered a broad-spectrum, first-line drug and may inhibit coronavirus infections and COVID-19. More clinical trials are needed to prove that GS-5734 (Remdesivir) is a safe and effective drug for the treatment of COVID-19.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.