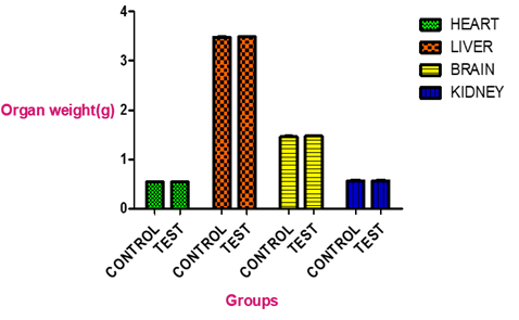Abstract
The aim of the present research is to investigate acute toxicity profiling of isolated Vigna mungo new natural polymer. Safety administration is the primitive criterion for any drug substance. To explore the safety and toxicity profiling of the novel polymer, this study was carried out. Vigna mungo novel polymer was isolated from the pulverised seeds of Vigna mungo which is part and parcel of our diet. This polymer is obtained using a non-solvent extraction method using acetone. Acute toxicity studies were performed according to the OECD guidelines 420. In this, the selected animal model is Swiss albino rats, grouped into control and test containing each three animals. 2000 mg/kg of Vigna mungo polymer was administered to a test group and did not produced any abnormalities and behavioural changes. Furthermore, histopathological studies, body weight, haematological parameters did not presented abnormal values. The observations found 2000mg/kg of a dose of the polymer did not cause lethality and death of any animal till 14 days of a period. It was concluded that Vigna mungo novel polymer is safe to administer up to 2000mg/kg dose. Hence, the novel Vigna mungo polymer is safer for therapeutic use in pharmaceutical formulations.
Full text article
References
Aneela, S., De, S., Kanthal, L., Choudhury, N., Das, B., Sagar, K. 2011. Acute oral toxicity studies of Pongamia Pinnata and Annona squamosa on albino wister rats. Int J Res Pharm Chem, 1(4):820– 824.
Anepu, S., Duppala, L., Nikhil, J., Devi, J. S. 2016. Formulation and evaluation of gastro retentive matrix tablets of Atenolol using melt granulation technique. International Journal of Pharmaceutical Sciences and Research, 7(3):1081–1081.
Anroop, B., Ghosh, B., Parcha, V., Vasanti, S. 2006. Studies on Ocimum gratissimum seed mucilage: Evaluation of binding properties. International Journal of Pharmaceutics, 325(1-2):191–193.
Ayodeji, A. E., Abubakar, A., Aliyu, N., Uhomoibhi, L. O., Garba, I. 2019. Acute and sub-acute toxicity of the crude extracts of the aerial parts of Daucus carota L. in laboratory rats. Journal of Medicinal Plants for Economic Development, 3(1):1–11.
Chunlaratthanaphorn, S., Lertprasertsuke, N., Srisawat, U., Thuppia, A., Ngamjariyawat, A., Suwanlikhid, N., Jaijoy, K. 2007. Acute and subchronic toxicity study of the water extract from the root of Citrus aurantifolia (Christm. et Panz.) Swingle in rats. Songklanakarin J Sci Technol, 29(1):125–164.
da Silva, R. O., Andrade, V. M., Rêgo, E. S. B., Dória, G. A. A., dos Santos Lima, B., da Silva, F. A., de Souza Araújo, A. A., de Albuquerque Júnior, R. L. C., Cardoso, J. C., Gomes, M. Z. 2015. Acute and sub-acute oral toxicity of Brazilian red propolis in rats. Journal of Ethnopharmacology, 170:66–71.
Divvela, H. N. D., Duppala, L., Kolapalli, V. R. M. 2016. Isolation and acute oral toxicity studies of Araucaria heterophylla novel natural polysaccharide gum in albino mice. World J Pharm Pharm Sci, 5(10):702–711.
Durga, D. H. N., Sowjanya, T. L., Pavani, T., Duppala, L. 2020. Formulation development and in-vitro evaluation of Molsidomine matrix tablets for colon specific release. Journal of Drug Delivery and Therapeutics, 10(2):59–68.
Gandhi, A., Verma, S., Imam, S. S., Vyas, M. 2019. A Review On Techniques For Grafting Of Natural Polymers And Their Applications. Plant Archives, 19:972–980.
Gil, E., Hudson, S. 2004. Stimuli-reponsive polymers and their bioconjugates. Progress in Polymer Science, 29:1173–1222.
Jain, N., Sharma, P., Sharma, N., Joshi, S. C. 2009. Haemato-biochemical profile following sub-acute toxicity of malathio i male albino rats. Avicenna J. Phytomed, 2:500–506.
Jothy, S. L., Zakaria, Z., Chen, Y., Lau, Y. L., Latha, L. Y., Sasidharan, S. 2011. Acute Oral Toxicity of Methanolic Seed Extract of Cassia fistula in Mice. Molecules, 16(6):5268–5282.
Liechty, W. B., Kryscio, D. R., Slaughter, B. V., Peppas, N. A. 2010. Polymers for Drug Delivery Systems. Annual Review of Chemical and Biomolecular Engineering, 1:149–173.
Pradeep, D. P., Murugan, K., Manoj, G. S. 2020. Evaluation of acute oral toxicity study of essential oils (Eos) from Pogostemon benghalensis and P. cablin in Wistar rats. Journal of Drug Delivery and Therapeutics, 10(3):142–147.
Qiu, Y., Park, K. 2001. Environment-sensitive hydrogels for drug delivery. Advanced Drug Delivery Reviews, 53:321–339.
Robinson, S., Chapman, K., Hudson, S., Sparrow, S., Spencer-Briggs, D., Danks, A., Hill, R., Everett, D., Mulier, B., Old, S., Bruce, C. 2009.
Sailaja, A. K., Amareshwar, P., Chakravarty, P. 2011. Different techniques used for the preparation of nanoparticles using natural polymers and their application. Int J Pharm Pharm Sci, 3(2):45–50.
Saleem, U., Amin, S., Ahmad, B., Azeem, H., Anwar, F., Mary, S. 2017. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Toxicology Reports, 4:580–585.
Santoshnaidu, M., Radha, G. V., Girish, P., Lohithasu, D. 2014. Comparison studies on transdermal films of natural tamarind seed polysaccharide extract containing anti-hypertension drug with PVA. HPMC and guar gum. World J Pharm Res, 3(5):753–763.
Shanmugam, S., Manavalan, R., Venkappayya, D., Sundaramoorthy, K., Mounnissamy, V. M., Hemalatha, S., Ayyappan, T. 2005. Natural polymers and their applications. Natural product, pages 478–481.
Shende, M. A., Marathe, R. P. 2016. Acute and Sub Chronic Oral Toxicity Studies of Hibiscus esculentus Mucilage on Swiss Albino Mice. Journal of Pharmaceutical Sciences and Research, 8(5):251–251.
Sholikhah, E. N., Mustofa, M., Nugrahaningsih, D. A. A., Yuliani, F. S., Purwono, S., Sugiyono, S., Widyarini, S., Ngatidjan, N., Jumina, J., Santosa, D., Koketsu, M. 2020. Acute and Subchronic Oral Toxicity Study of Polyherbal Formulation Containing Allium sativum L., Terminalia bellirica (Gaertn.) Roxb., Curcuma aeruginosa Roxb., and Amomum compactum Sol. ex. Maton in Rats. BioMed Research International, 2020:1–18.
Sravani, B., Deveswaran, R., Bharath, S., Basavaraj, B. V., Madhavan, V. 2011. Studies on Vigna mungo mucilage as a pharmaceutical excipient. Journal of chemical and pharmaceutical research, 3(2):118– 143.
Walum, E. 1998. Acute oral toxicity. Environmental health perspectives. No OT. 425. OECD guidelines for the testing of chemicals, section, 106(2):497– 503.
WHO 1993. Research Guidelines for Evaluating the Safety and Efficacy of Herbal Medicines. World Health Organization. World Health Organization, pages 94–9290611103.
Yadav, I. K., Jaiswal, D., Ghosh, N., Singh, H. P., Mishra, A., Bhattacharya, A., Bajpai, M. 2009. Evaluation of Seed Flour of Vigna mungo (L.) based Sustained Release Matrix Tablets of Diclofenac Sodium. Journal of Pharmacy Research, 2(5):834–838.
Yassine, E. Z., Dalila, B., Latifa, E. M., Smahan, B., Lebtar, S., Sanae, A., Abdellah, F. 2016. Phytochemical screening, anti-inflammatory activity and acute toxicity of hydro-ethanolic, flavonoid, tannin and mucilage extracts of Lavandula stoechas L. from Morocco. Int J Pharm Phytochem Res, 8(1):31–37.
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.