Abstract
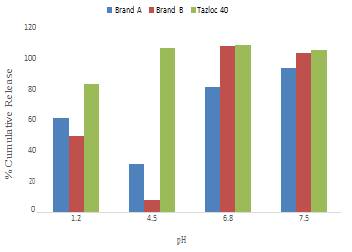
Telmisartan is Angiotensin II Receptor Antagonist, which is used in the prevention and treatment of Hypertension. Telmisartan belongs to class II drug in BCS classification, i.e. low solubility and high permeability. The dissolution of a drug in specific gastrointestinal fluids is important for absorption into the systemic circulation. Dissolution studies {using USP Apparatus 2 (Paddle Apparatus) at 75 rpm with dissolution media including pH 1.2 hydrochloric acid, and pH 4.5, pH 6.8, pH 7.5 buffers} on various commercial products of Telmisartan were carried out to compare drug release. Statistical methods were used to compare the dissolution profiles. These included Model independent method, i.e. Dissimilarity factor (f1) and the Similarity factor (f2) as recommended by FDA. It was observed that formulation factors significantly affect the solubility of a drug at various pH. The dissolution profile of Tazloc tablets at various pH exhibited significant differences compared to other commercial products. Tazloc tablets showed consistently higher release at pH 4.5 and 7.5, (i.e., pH conditions relevant to the intestine) suggesting its pharmacokinetic activity could be perhaps superior to other marketed brands as higher dissolution leads to better absorption that may lead to better plasma levels and thus better efficacy.
Full text article
Generated from XML file
Authors
Rita R. Lala, & Amol S. Shinde. (2023). Dissolution studies of telmisartan: Influence of pH on the release of drug from oral formulations. International Journal of Research in Pharmaceutical Sciences, 9(4), 1248–1253. Retrieved from https://ijrps.com/home/article/view/4450
Copyright (c) 2018 International Journal of Research in Pharmaceutical Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.