Abstract
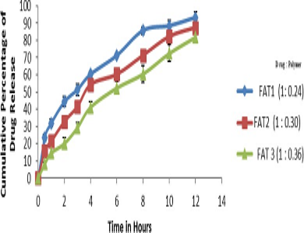
Stomach Specific Floating Tablets (SSFT) with a combination of Amoxicillin-Trihydrate (AT) and Ranitidine Hydrochloride (RH) were developed by using different grades of Hydroxypropylmethylcellulose (HPMCK) (i.e.HPMCK 100M, HPMCK4M and HPMCK15M), to treat patients with H. pylori-infected duodenal ulcer. Floating tablets were prepared by direct compression method, developed formulations were evaluated for different pre-compression and post-compression parameters like angle of repose, compressibility index, hardness, weight variation, floating lag time, content uniformity, and in-vitro drug release. In-Vitro release of two drugs (Amoxicillin-Trihydrate and Ranitidine hydrochloride) from the developed formulation was estimated by the Simultaneous Estimation method (Vierordt's Method). The optimized formulation was subjected to Radio graphical evaluation by incorporating the BaSO4, a radio-opaque substance by replacing a part of the drug from the optimized formulation of into the formulation and then it was administered to the healthy human volunteers to find out the in-vivo residence time. In-vivo X-ray studies were conducted both in fed condition, as well as fasted condition the optimized formulation showed a gastric residence time of more in fed state than that of fasting state. From these studies it was clearly observed that the floating tablets should be given to patients after a standard food and with frequent intake of water.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.