Development and Validation of Simple UV Spectrophotometric Method for the Determination of Pretomanid
Abstract
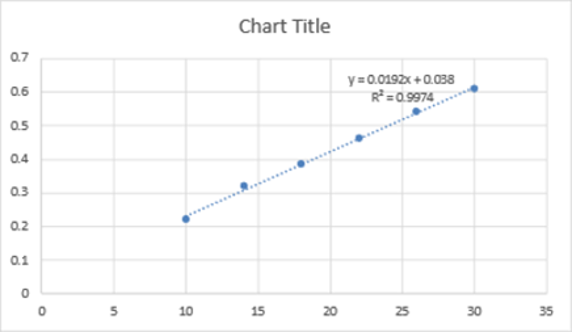
In order to treat multidrug resistant TB, pretomanid, a nitroimidazooxazine antimycobacterial agent, is used with other antituberculosis medications. There is no technique for its analysis that uses spectroscopy, HPLC or HPTLC. Since a UV spectrophotometric approach for Pretomanid analysis must be developed. Utilizing a Shimadzu UV-2600, a quick, accurate, straightforward, and affordable UV spectrophotometric approach has been devised to determine Pretomanid. Solvent made of methanol to assess the bulk Pretomanid concentration. The detection process was placed at a wavelength of 321 nm. The parameters linearity, accuracy, precision, ruggedness, and robustness were taken into consideration during method validation in accordance with ICH Q2R1 criteria, as well as LOD and LOQ. It demonstrated linearity in the 10– 30(g/mL) range at a predetermined max of 321 nm, and had a strong correlation coefficient (R2-0.997) and outstanding mean recovery (99.00–100.07%). In terms of intraday and interday precision, Pretomanid’s% RSD was discovered to be 0.6366 and 0.666, respectively. Pretomanid identification using this approach was effective. The method’s linearity, accuracy, repeatability, and reproducibility were statistically and empirically verified. The out- comes demonstrated the method’s applicability for both routine examinations of protomanid bulks and industrial formulations. The suggested UV-Vis Spectrophotometric approach was verified in accordance with ICH requirements and found to be simple, accurate, precise and quick for the determination of Pretomanid.
Full text article
References
A Bahuguna and D S Rawat. An overview of new antitubercular drugs, drug candidates, and their targets. Medicinal research reviews, 40(1):263–292, 2020.
U Parveen, S Sultana, S F Heba, R Rafi, A Begum, and N Fatima. Pretomanid: A novel therapeutic paradigm for the treatment of drug-resistant tuberculosis. Indian Journal of Tuberculosis, 68(1):106–113, 2021.
U.S. Food and Drug Administration. FDA briefing document: Briefing Document: Pretomanid Tablet, 200 mg, 2021. Meeting of the Antimicrobial Drugs Advisory Committee (AMDAC). June 06, 2019. Accessed on April 12, 2021.
C K Stover, P Warrener, D R Vandevanter, D R Sherman, T M Arain, M H Langhorne, and W R Baker. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature, 405(6789):962–966, 2000.
AMDAC. FDA Briefing Document Pretomanid Tablet, 200 mg Meeting of the Antimicrobial Drugs Advisory Committee (AMDAC), 2019. Accessed on June 06, 2019.
S Tyagi, E Nuermberger, T Yoshimatsu, K Williams, I Rosenthal, N Lounis, and J Grosset. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrobial agents and chemotherapy, 49(6):2289–2293, 2005.
A J Lenaerts, V Gruppo, K S Marietta, C M Johnson, D K Driscoll, N M Tompkins, and I M Orme. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrobial agents and chemotherapy, 49(6):2294–2301, 2005.
U Manjunatha, H I Boshoff, and C E Barry. The mechanism of action of PA-824: novel insights from transcriptional profiling. Communicative & integrative biology, 2(3):215–218, 2009.
R Singh, U Manjunatha, H I Boshoff, Y H Ha, P Niyomrattanakit, R Ledwidge, and C E Barry 3rd. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science, 322(5906):1392–1395, 2008.
ICH Harmonised Tripartite Guideline. Validation of analytical procedures: text and methodology, in International Conference on Harmonization (ICH), Q2(R1), 2005. IFPMA, Geneva, Switzerland.
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.