Abstract
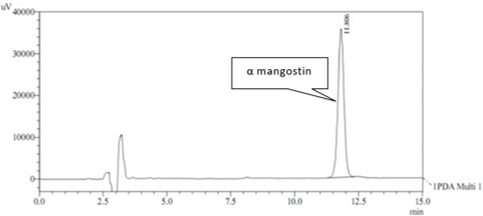
Kandis, Garcinia cowa Roxb, traditionally has been used for many purposes. Many parts of G. cowa have been used in traditional folk medicine as antipyretic and anti-inflammatory. α-mangostin represents the majority of the clinical benefits of this herbal medicine. It is reasonable and logical to determine the concentration of α-mangostin as a chemical marker for the quality control of G. cowa and its products. The aim of this study was to set up a validated and stability-indicated isocratic reverse phase high performance liquid chromatographic (RP- HPLC) method for quality control and quantity determination of α-mangostin from ethanol extract of G. cowa. The assay was fully validated and shown to be linear (r2 = 0.999), sensitive (LOD = 0.04 μg/ml and LOQ = 0.16 μg/ml) and precise (intra-day variation ≤ 1.6 %, inter-day variation ≤ 4.3%). Accuracy of the method was determined by a recovery study conducted at 3 different levels, and the average recovery was 86.67%. Total analysis was ~ 15 min. The present method should be useful for analytical research and for routine quality control analysis of α-mangostin in ethanol extract of G. cowa.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.