Abstract
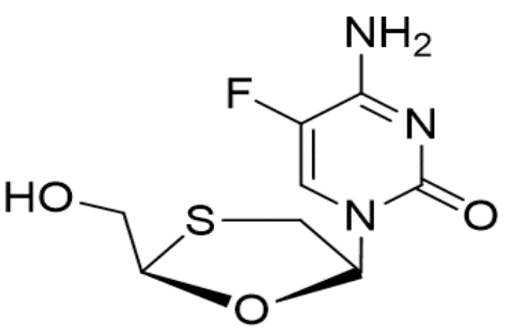
A simple, expeditious, and explicit stability-indicating High-Performance Liquid Chromatography (HPLC) analytical test method was developed for the quantitative analysis of Emtricitabine, Tenofovir, and Rilpivirine in bulk drugs and combined dosage formulations. Using the ICH guidelines method was validated using a C18 column of size 250 mm X 4.6 mm, 5µ, maintained at 25oC. The total run time maintained was 10 min. Acetonitrile and 0.1% TEA prepared in water adjusted with pH 3.0 was adapted as a mobile phase. The injection volume of samples was 20μL and UV-DAD detector system set at 265 nm used for UV detection. As per the ICH guidelines method was validated. The retention times were observed as 2.52, 3.27, 6.70 min for Emtricitabine, Rilpivirine, and Tenofovir disoproxyl fumarate, respectively. Linearity ranges were observed 24-56 µg/mL Emtricitabine, 3-7 µg/mL Rilpivirine and 30-70 µg/mL Tenofovir. Relative Standard Deviation not exceed 2%. In contract to the conventional method of HPLC, a method developed was able to give better resolution of swift retention times in the separation of various degradation products along with the pure active pharmaceutical ingredients. The proposed method exhibited excellent reproducibility and repeatability. The stress studies performed by following ICH guidelines indicated that the present HPLC method is explicit and stability-indicating. Since the present HPLC method has the capacity to separate the drugs with high resolution in tablet dosage forms, hence the method can be exploited for routine analysis of quality control sample and stability analysis.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.