Abstract
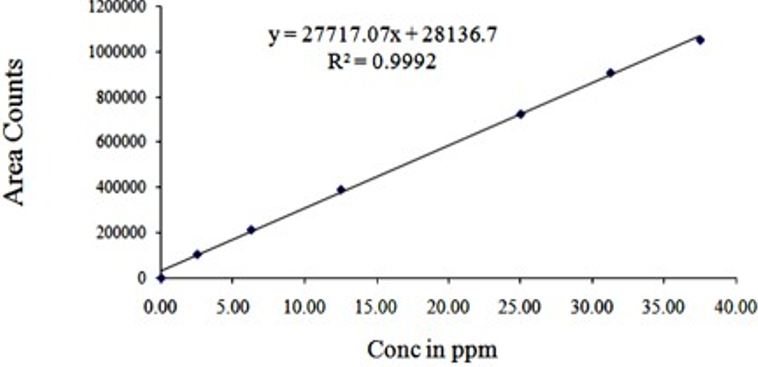
We have developed a completely unique and reliable HPLC technique for simultaneous quantification of Lopinavir and Rilpivirine. Chromatographic detachment was attained on a X-bridge phenyl column (150x4.6mm, 3.5 µ) using isocratic elution with a buffer containing buffer and acetonitrile with the proportion of 70:30 as movable phase with a flow of 1 ml/min at room temperature and UV detection was carried out at 250 nm. Dissolve 1ml of tri ethylamine in 1 lt of HPLC grade water and filter through 0.45 µ filter paper, this solution was used as buffer. 8 min. run time was used to separate Lopinavir and Rilpivirine. Analysis was achieved within 15 min over an honest linearity within the concentration range from 20-300 µg/ml of Lopinavir and 2.5-37.5 µg/ml of Rilpivirine. By injecting the standard six times, system suitability parameters were studied and the outcomes were under the acceptable limit. Precision and recovery study results were found to be within the suitable limit. By using the above technique, assay of Lopinavir and Rilpivirine was performed and found to be within the limit. Degradation studies were carried out on Lopinavir and Rilpivirine, with a purity threshold greater than purity angle in all conditions and within the allowable range. The above mentioned technique was validated according to ICH guidelines.
References
Boyer, P. L., Sarafianos, S. G., Arnold, E., Hughes, S. H. 2001. Selective Excision of AZTMP by Drug-Resistant Human Immunodeficiency Virus Reverse Transcriptase. Journal of Virology, 75(10):4832–4842.
Brignone, N. F., Pozner, R. E., Denham, S. S. 2019. Origin and evolution of Atriplex (Amaranthaceae s.l.) in the Americas: Unexpected insights from South American species. TAXON, 68(5):1021–1036.
Capparelli, E. V., Holland, D., Okamoto, C., Gragg, B., Durelle, J., Marquie-Beck, J., van den Brande, G., Ellis, R., Letendre, S. 2005. Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV. AIDS, 19(9):949– 952.
Eisinger, R. W., Dieffenbach, C. W., Fauci, A. S. 2019. HIV Viral Load and Transmissibility of HIV Infection. JAMA, 321(5):451–452.
Gardner, E. M., Burman, W. J., Steiner, J. F., Anderson, P. L., Bangsberg, D. R. 2009. Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS, 23(9):1035–1046.
Goebel, F., Yakovlev, A., Pozniak, A. L., Vinogradova, E., Boogaerts, G., Hoetelmans, R., de Béthune, M. P. P., Peeters, M., Woodfall, B. 2006. Short-term antiviral activity of TMC278 – a novel NNRTI – in treatment-naive HIV-1-infected subjects. AIDS, 20(13):1721–1726.
Kwong, K. C. N. K., Mehta, A. R., Nedergaard, M., Chandran, S. 2020. Defining novel functions for cerebrospinal fluid in ALS pathophysiology. Acta Neuropathologica Communications, 8(1):1–18.
Mordant, C., Schmitt, B., Pasquier, E., Demestre, C., Queguiner, L., Masungi, C., Peeters, A., Smeulders, L., Bettens, E., Hertogs, K., Heeres, J., Lewi, P., Guillemont, J. 2007. Synthesis of novel diarylpyrimidine analogues of TMC278 and their antiviral activity against HIV-1 wild-type and mutant strains. European Journal of Medicinal Chemistry, 42(5):567–579.
Orešković, D., Klarica, M. 2014. A new look at cerebrospinal fluid movement. Fluids and Barriers of the CNS, 11(1):1–3.
Ren, J., Nichols, C., Bird, L., Chamberlain, P., Weaver, K., Short, S., Stuart, D. I., Stammers, D. K. 2001. Structural mechanisms of drug resistance for mutations at codons 181 and 188 in HIV-1 reverse transcriptase and the improved resilience of second generation non-nucleoside inhibitors 1 1Edited by J. Karn. Journal of Molecular Biology, 312(4):795–805.
Stellbrink, H. J. 2007. Antiviral drugs in the treatment of AIDS: what is in the pipeline? European Journal of Medical Research, 12(9):483–495.
Venning, G. R. 1982. Validity of anecdotal reports of suspected adverse drug reactions: the problem of false alarms. BMJ, 284(6311):249–252.
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.