Abstract
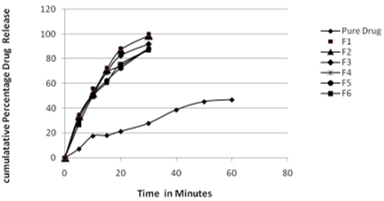
Carbamazepine is a common antiepileptic drug and due to its poor aqueous solubility absorbed very slowly and erratically after oral administration. To enhance its solubility, dissolution rate self microemulsifying drug delivery system (SMEDDS) was formulated and evaluated. The solubility of carbamazepine was determined in various vehicles. Pseudoternary phase diagrams were evaluated for microemulsification existence area, and the release rate of carbamazepine was investigated using an in vitro dissolution test. SMEDDS formulations were tested for microemulsifying properties and the resultant microemulsions were evaluated for clarity, precipitation and particle size distribution. Formulation development and screening was done based on results obtained from phase diagrams and characteristics of resultant microemulsion. The optimized formulation was studied for in vitro study and was found that the formulation containing Labrafac CC (28.5%), Tween 60 (53.64%) propylene glycol:ethanol (1:1) showed a complete release in 30 minutes as compared with the pure drug which showed a limited dissolution rate. The stability studies found that the formulations were stable over period of 3 months. Thus the study confirmed that the SMEDDS formulation can be used as a possible alternative to traditional oral formulation of carbamazepine to improve its solubility and dissolution rate.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.