Abstract
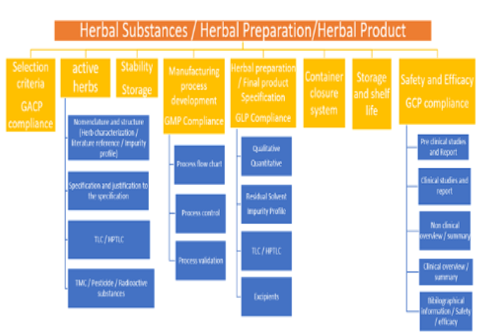
Recently, herbal medicinal products (HMP) have gained importance and are extensively used in the prevention and treatment of various ailments. A commercial herbal medicinal product should comply with the regulatory requirements of quality, safety and efficacy. Currently, the standards and regulations of herbal medicinal products are varying from country to country, which poses a challenge to the manufacturing companies to place a standardized herbal product in the global market. Hence a collaborative effort must be taken both by regulatory bodies and the World Health Organization (WHO) to establish harmonized regulations for a herbal medicinal product. An attempt has been made in this review which may pave the way to meet out the constraints and challenges in the manufacturing and marketing of herbal medicinal product worldwide.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.