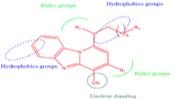
Abstract
For the identification of the lead compounds, a molecular docking tool is used. The little structure, namely Ligand, generally holds together the protein places. It describes a similar approach that utilizes to place over another three-dimensional structure of a probable drug on its prospective object sites. Given that, it was worthwhile to build a virtual library of benzimidazole derivatives to find lead structures to test against C. Albicans. The two-dimensional structure of all planned compounds was drawn by using the current version software and pass on to the software window. The energy of all three-dimensional structures was reduced by Molecular Orbital Package up to Root mean square gradient 0.001 and put aside in MDL Molfile (.Mol) format. To assess the likely potential of the Quantitative Structure-Activity Relationship models, the dataset was split into a training set comprising of 32 molecules and a test set of 8 molecules in such a way that the structural variety and an extensive range of biological action in the specific set were added. The IC50 values were transformed to pIC50 to give numerically larger data values.
Full text article
References
Drumm, J.E., Deininger, D.D. 2007. Facile Preparation of Fused Ring Azolylureas. Tetrahedron letters, 48(31):5535–5538.
Furniss, B.S., Hannaford, A.J., Tatchell, A.R. 1989. Vogel’s Textbook of Practical Organic Chemistry, 5th Edn, Pearson Education. page 1514, London. ISBN: 9788177589573.
Kazimierczuk, Z., Shugar, D. 1989. Preparation and Properties of the 5,6- and 4,6(5,7)-Dinitro Derivatives of Benzimidazole and their 1-β-D-Ribofuranosides. Nucleosides and Nucleotides, 8(8):1379–1385.
Kazimierczuk, Z., Upcroft, J.A., Upcroft, P., Górska, A., Starościak, B., Laudy, A. 2002. Synthesis, antiprotozoal and antibacterial activity of nitro- and halogeno-substituted benzimidazole derivatives. Acta Biochimica Polonica, 49(1):185–195.
Kimura, T., Takase, Y., Hayashi, K. 1993. Structure- Activity Relationship of N- [2- (Dimethy1amino)-6-[3-(5-methyl-4-phenyl-1H-imidazol-1-yl) propoxy] phenyl]-N’-pentylurea and Analogues. Novel potent inhibitors of acyl-CoA: cholesterol O-acyltransferase with antiatherosclerotic activity. J Med Chem, 36(11):1630–1640.
Li, H.-Y., DeLucca, I., Boswell, G.A., Billheimer,J.T., Drummond,S., Gillies, P.J.,Robinson, C. 1997. Design, synthesis and structure-activity relationship studies of novel 4,4- bis(trifluoromethyl)imidazolines as acyl-CoA: Cholesterol acyltransferase (ACAT) inhibitors and antihypercholesterolemic agents. Bioorganic & Medicinal Chemistry, 5(7):1345–1361.
Nakao, K., Kubota, H., Yasuhara, M. 2001. Novel Hydroxyphenylurea Dual inhibitor Against Acyl-CoA: cholesterol Acyltransferase (ACAT) and Low-Density Lipoprotein (LDL) Oxidation as Antiatherosclerotic Agent. Bioorganic & medicinal chemistry, 9(4):853–861.
Purchase, C.F., White, A.D. 1996. Tetrazole-substituted ureas as inhibitors of Acyl-CoA: Cholesterol O-Acyltransferase (ACAT). Bioorganic & medicinal chemistry letters, 6(15):1753–1758.
Purchase, T.S., Holmes, A. 1997. Inhibitors of Acyl- CoA: Cholesterol Acyltransferase: Novel Trisubstituted Ureas as Hypocholesterolemic agents. Bioorganic & medicinal chemistry, 5(4):739–747.
Robl, J.A., Sulsky, R., Sun, C., Simpkins, L.M., Wang, T., Dickson, J.K. 2001. A Novel Series of Highly Potent Benzimidazole-Based Microsomal Triglyceride Transfer Protein Inhibitors. J. Med. Chem, 44(6):851–856.
Roth, B.D., Roark, W. H., Picard, J.A., Stanfield, R.L., Bousley, R.F., Anderson, M.K., Hamelehle, K.L., Homan, R., Krause, B.R. 1995. Inhibitors of acyl-coa:cholesterol acyltransferase (ACAT). 15. sulfonylurea inhibitors with excellent hypocholes terolemic activity in vivo. Bioorganic & Medicinal Chemistry Letters, 5(20):2367–2370.
Tanaka, A., Terasawa, T., Hagihara, H., Sakuma, Y., Ishibe, N., Sawada, M., Takasugi, H., Tanaka, H. 1998. Inhibitors of acyl-CoA: cholesterol O-acyltransferase (ACAT). Part 1: Identification and structure-activity relationships of a novel series of substituted N-alkyl-N-biphenylylmethyl-N′-arylureas. Bioorganic & Medicinal Chemistry, 6(1):15–30.
Terasawa, T., Tanaka, A., Hagihara, H., Sakuma,Y., Ishibe, N., Sawada, M., Takasugi, H., Tanaka, H. 1998. Inhibitors of Acyl-CoA:CholesterolO-Acyltransferase 2.Identification and Structure Activity Relationships of a Novel Series of N-Alkyl-N-(heteroaryl-substituted benzyl)-N‘-arylureas1. Journal of Medicinal Chemistry, 41(13):2390–2410.
Tiwari, A.K. 2006. Synthesis and antiviral activities of N-substituted 2-substituted-benzimidazole derivatives. Indian journal of chemistry, 45(2):489–493.
White, A.D., Creswell, M.W., Chucholowski, A.W., Blankley, C.J., Wilson, M.W., Bousley, R.F., Essenburg, A.D., Hamelehle, K.L., Krause, B.R., Stanfield, R.L., Dominick, M.A., Neub, M. 1996. Hetero-cyclic Ureas: Inhibitors of Acyl-CoA:CholesterolO-Acyltransferase as Hypocholesterolemic Agents1. Journal of Medicinal Chemistry, 39(22):4382– 4395.
Wilde, R.G., Billheimer, J.T., Germain, S.J., Gillies, P. J., Higley, C.A., Kezar, H.S., Maduskuie, T.P., Shimshick, E.S., Wexler, R.R. 1995. Acyl CoA:cholesterol acyltransferase (ACAT) inhibitors: ureas bearing heterocyclic groups bioisosteric for an imidazole. Bioorganic & Medicinal Chemistry Letters, 5(2):167–172.
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.