Abstract
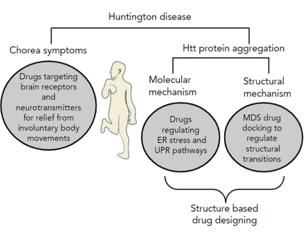
Huntington disease (HD) is a fatal and progressive neurodegenerative disease that has affected the social and personal life of patients. The disease causes the most disturbing symptoms of chorea, which is characterized by uncontrolled body movements. HD patients are being treated by providing drugs that maintain neurotransmission balance and relieve chorea symptoms. HD has been associated with mutant Huntingtin protein (mHtt) with more than thirty-six polyQ stretches at N terminal of 34 kDaHtt protein. mHtt protein undergoes misfolding, which leads to accumulation of toxic mHtt aggregates in the brain. The phenomenon of protein aggregation initiates a cascade of events, eventually leading to endoplasmic reticulum (ER) stress and misregulated unfolding protein response (UPR). Different molecular targets have been identified from ER stress and UPR pathways for finding potential molecules that can treat HD. Overall, the mechanism causes structural transitions in mHtt, which can be controlled at the subatomic and molecular level by molecular dynamic simulations (MDS). The MDS strategies help to observe structural changes in the mHtt protein and association pattern between the protein and novel drug compounds. Hence, this study explains the journey of HD research to computational strategies and the scope of structural drug designing in psychologically disturbing Huntington’s disease.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.