Abstract
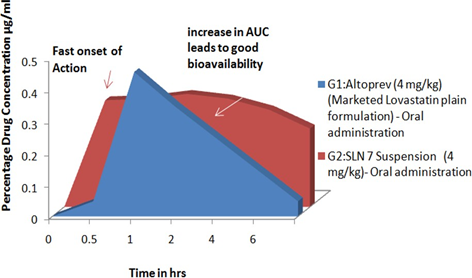
The purpose of this research is to increase bioavailability by solid lipid nanoparticle (SLN) carrier for low bioavailable drugs (< 5%) such as Lovastatin. Eight SLN loaded Lovastatin was designed and optimised by variables such as Particle Size (PS in nm) and Zeta Potential (ZP in mV) using a micro emulsification technique. SLN 7 was chosen as the optimised formulation according to the findings obtained and the same was chosen for invivo pharmacokinetic and triton-induced antihyperlipidemic operation. SLN7 confirms an improvement in bioavailability of 3.15 percent by an improvement in AUC compared to conventional dosage type (Altoprev) from the pharmacokinetic invivo results. SLN was also an appropriate career in drug delivery for Lovastatin by enhancing bioavailability and therapeutic response. The stability studies of SLN7 revealed that the evaluation parameters of SLN did not change significantly. It was verified from the data that the drug-loaded SLN was stable under varying temperature and humidity conditions. While compare to 25˚ c±2˚c/ 60% RH, SLN are more stable in 4˚ c±2˚ c and shows good reproducible reports in Particle Size (nm), Zeta potential (mV), PI and EE% data. Therefore, Solid Lipid Nanoparticle is a viable drug carrier mechanism for low bioavailable Lovastatin to improve their bioavailability through efficiently permeating them.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.