Abstract
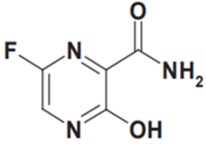
Due to its high transmission potential, COVID-19 disease has turned out to be a worldwide public health threat. As of May 2020, over 5M infections and 300,000 deaths had been reported globally. At the moment, there is limited research evidence from RCTs recommending certain drugs for the treatment of suspected or confirmed COVID-19 patients. However, a number of studies have proposed numerous antiviral agents as a potential treatment option based on experiments done on animal models infected with other viral diseases. One such drug is favipiravir. The purpose of this review, therefore, was to examine recent updates about favipiravir and its likely role in the treatment of coronavirus disease. As has been previously reported in literature, favipiravir acts as a broad-spectrum medication that prevents the multiplication of flavivirus, filovirus, poliovirus, arenaviruses, and rhinovirus. The drug has recently been reported in some studies as useful in shortening the time of clinical recovery for COVID-19 patients. The study guaranteeing the usefulness of favipiravir in the treatment of the virus has since been withdrawn temporarily. The analysis in the study was largely open-label and non-randomized. Even with its adverse pharmacokinetic profile and the inconclusive data regarding its usefulness in the management of COVID-19, China has authorized the drug as a suitable treatment of COVID-19 patients as of March 2020. Still, favipiravir has been included in a number of ongoing trials, together with other antiviral drugs, such as lopinavir/ ritonavir.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.