Abstract
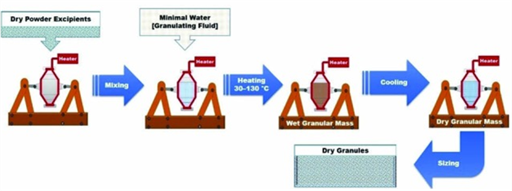
Progress in pharmaceutical research has delivered very strong medications, which require cautious definition and creation so as to deliver strong oral measurement structures with satisfactory homogeneity and physical stability. Mixing and formulation of low dose medications can be individually testing because of issues identified with isolation, content uniformity and physical stability. A cautious control on these variables is vital while fabricating low dose drugs. Choice of excipients for the particular strides during detailing/process improvement is basic to build up a homogenous and isolation free low portion definition. Numerous sorts of categories of gear have been intended towards encourage blending of low portion drugs with excipients. A side from traditional detailing strategies (like wet granulation, dry granulation, and direct compression) different new procedures have been announced, for example, high shear granulation, requested blending and spry drying. These innovative headways ensure enhanced the assembling and nature of low dose drugs items by accomplishing their predefined goal of substance consistency through outblending and plan of low dose drugs. Blending and detailing of low portion drugs are advanced work and includes part of issues related to isolation, content consistency and physical soundness which can be constrained by right choice of material, strategy and machine. This article surveys current headways identified with plan systems of low dose drugs.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.