Abstract
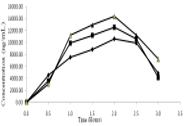
A rapid, sensitive and selective bioanalytical method was developed and validated by Liquid Chromatography - Mass spectrometry (LC-MS/MS) for determination and comparison of Selexipag% assay in various biological materials. Selexipag was extracted and compared its % assay after protein precipitation technique from various biological materials such as rat plasma, rabbit plasma, human plasma and urine. Ambrisentan was selected as internal standard. Selected analytical column Waters, X-Bridge C18 3.5µ (150 x 4.6 mm), mobile phase consists of Hexane sulfonic acid and Acetonitrile (80:20 v/v) at a flow rate of 1.0 mL /min in isocratic mode and Selexipag was determined by the +ve mode of electrospray ionization by using Mass detector. The method was developed to assess the lower limit of detection (LLOD)(0.5 ng/mL), lower limit of quantification(LLOQ) (5 ng/mL) concentrations and Linearity range of 1 ng/mL to 20 ng/mL concentration with regression correlation coefficient 0.999 were observed for Selexipag in Rat plasma. The test samples at lower, medium and higher concentrations of Selexipag shows precision (% CV was 0.8 to 1.11) and accuracy results (97.3 % to 100.6%) for inter-day and intra-day analysis at 1, 5, 10, 15 ng/mL concentrations of Selexipag. Stability of Selexipag exists in all conditions like wet extract, bench top, freeze-thaw and in instrument auto sampler as per FDA guidelines.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.