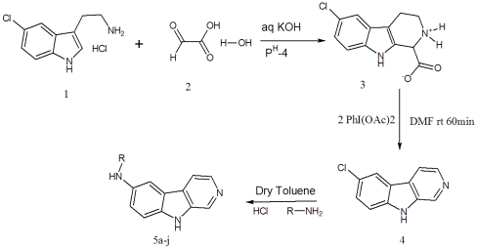Abstract
β-Carboline is also known as nor-harmane. It is a nitrogen-containing heterocyclic compound formed in plants and animals as Maillard reaction products between amino acids and reducing sugars or aldehydes. These tricyclic nitrogen heterocyclics play a vital role in medicinal chemistry, due to significant biological activities of their derivatives. It is also a key pharmacophore present in a large number of natural tricyclic alkaloids. Current work is reported with the synthesis and antibacterial activity screening of a new series of N- Substituted-9H-β-carboline-6-amine derivatives. The title compounds were synthesized according to the well known Pictet Spengler reaction in three steps by taking 5-Chlorotryptamine and glyoxalic acid as starting materials. This is an acid-catalyzed intramolecular condensation of an iminium ion and an aromatic C-nucleophile which resulted in the formation of 6-Chloro-1,2,3,4- tetrahydro-9H-pyrido[3,4-b]indol-2-ium-1-carboxylate (3). Oxidative decarboxylation and aromatization of compound 3 with iodobenzene diacetate led to the 6-Chloro-β–carboline (4) which were treated with different mono substituted amines gave the title compounds (5 a-J). Structures of the synthesized entities were confirmed spectroscopically (FT-IR, 1H NMR and Mass) and screened for antibacterial activity against various pathogenic bacterial strains (Streptococcus pyogenes, Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa) by disc diffusion method. The title compounds showed moderate to good antibacterial activity.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.