Abstract
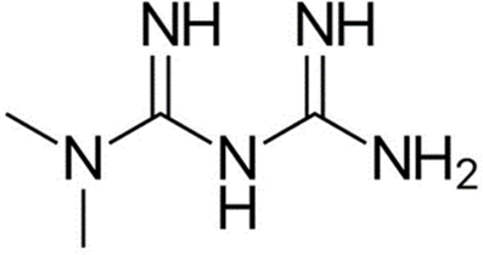
A Specific, Linear and Precise reversed phase- HPLC was developed for the simultaneous estimation of Metformin HCl and Empagliflozin and the column used is Zorbax SB Phenyl with length, Internal diameter and Particle size of 250mm, 4.6 mm and 5µm respectively. The Mobile phase is Phosphate buffer: ACN: Methanol in ratio 45:25:30. 1.0 ml/min was the used flow rate and the wavelength was adjusted to 220nm for detection. The retention time for Empagliflozin was found to be 5.5min and for Metformin was 9.3min. Both the APIs exhibited good linearity revealing correlation coefficient(R) of 0.9999. The percentage recoveries for Metformin and Empagliflozin was found to be 100.0 – 100.9% and 100.3 – 102.4% respectively which was found to be within the limit. Forced degradation studies were performed and the developed method has suitable specificity as no interference is observed with impurity spiked sample and placebo of Drug Product. The proposed drug products were subjected to various types of stress conditions according to ICH Q1 guidelines like acidic, alkaline, neutral, peroxide, and Thermal conditions. The degradation products were well resolved from the main peaks , thus indicating the stability indicating nature of the method. The method was validated with respect to system suitability, linearity, accuracy, precision and robustness according to ICH guidelines and the proposed RP-HPLC Method was accurate, precise and linear for the simultaneous determination of Metformin and Empagliflozin in bulk and pharmaceutical formulations.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.