Abstract
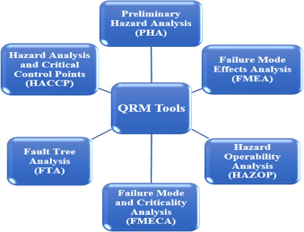
Risk in general means exposure to harm or a factor that can contribute towards bringing harm to a system. Almost every operation in the pharmaceutical industry is susceptible to risks. There is a need to overcome risks and minimise them to prevent unwanted changes in product quality and safety. This prevention can be done by carrying out quality risk management (QRM) that facilitates the proper characterisation of risks, risk analysis, risk assessment to control and reduce risks. ICH Q9 guidelines explain quality risk management and its applications in the pharmaceutical industry. Cleaning validation is performed to verify and evaluate the efficacy of cleaning procedures used to clean the equipment after production and to prevent cross-contamination between products that are manufactured in the production facility. The current study focusses on reviews of quality risk management and cleaning validation policies. By incorporating QRM in the quality policy, companies can look to improve the modern Approach towards facing risks related to the issues that affect the product quality, safety and compliance concerning the cleaning of equipment used in manufacturing and supporting operations in the industries.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.