Abstract
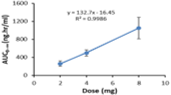
Doxazosin is used for treating symptoms of benign prostatic hyperplasia (BPH). Besides, it is also prescribed for patients with mild to moderate essential hypertension. The object of the current study was to assess the linearity in the pharmacokinetics of doxazosin after administration of doxazosin as a single dose tablet containing 2, 4 and 8 mg doxazosin mesylate. Thirty Iraqi healthy male adult subjects were given 2, 4 and 8 mg doxazosin mesylate tablet in a randomized, cross-over, open-label, fasting, three-period, three-sequence design separated by one week wash out interval between dosing. Serial blood samples were obtained from each subject before drug intake (zero time) and then at 0.33, 0.67, 1.0, 1.33, 1.67, 2.0 , 2.5, 3.0, 4.0, 6.0, 8.0, 12.0, 24.0, 36, 48, 60, and eventually at 72 hours after dosing. The pharmacokinetic parameters Cmax, AUC0–t, AUC0–∞, Tmax and Thalf were determined from plasma concentration-time data of the drug by non-compartmental analysis. Statistical analysis of doxazosin pharmacokinetic parameters obtained after administration of the investigated dose ranges 2-8 mg demonstrated linear pharmacokinetics.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.