Abstract
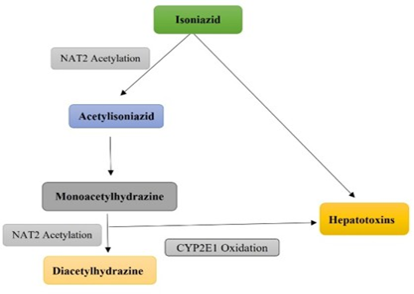
Tuberculosis remains a leading cause of death in developing countries. In India antitubercular treatment was effective from the 1950s and has been engaged for more than 50 years. Hepatotoxicity secondary to antitubercular drug treatment is a major threat to tuberculosis. Anti-tubercular drugs like isoniazid, rifampicin, and pyrazinamide are potentially hepatotoxic and their adverse reactions are based on dose or hypersensitivity. Hepatotoxicity can occur within a few weeks of initiating intensive phase and elevation of serum transaminase levels with signs and symptoms are considered as the diagnostic criteria. The variations in the incidence of anti-tubercular drug-induced hepatotoxicity may be due to differences in patient characteristics, regimen used, type of monitoring and the diagnostic criteria defining hepatotoxicity. Predisposing factors associated with anti-tubercular drug-induced hepatotoxicity include advanced age, gender, nutritional status, alcohol intake, extensive TB, indiscriminate use of various drugs, ethnic factors, chronic liver disease and concomitant infections. There are no evidence-based guidelines that are accessible for the management of these patients. Management of hepatotoxicity generally involves withholding the anti-tubercular drugs and reintroduction after normalization of biochemical markers of liver injury.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.