Abstract
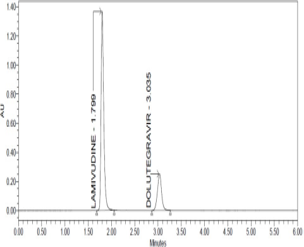
Dovato tablets (lamivudine and dolutegravir combination) are full therapy regimen for the Type 1 human immunodeficiency virus (HIV-1) infection in adults without history of antiretroviral therapy. The aim of present research focused on development of validated stability indicating method for the quantification of lamivudine and dolutegravir in combined dosage form.The separation was achieved on a Cosmicsil C18 column. The mobile phase consisted of 0.1% orthophosphoric acid (pH 3.5) – acetonitrile (50:50, v/v). Photodiode array detector was used to detect the analytes at 258 nm. The method performance was validated in compliance with the recommendations of the International Conference on Harmonization. The method was validated with selectivity, limit of detection (0.262 μg/ml for lamivudine and 0.238 μg/ml for dolutegravir), linearity (150-450 μg/ml for lamivudine and 25-75 μg/ml for dolutegravir), limit of quantification (0.874 μg/ml for lamivudine and 0.793 μg/ml for dolutegravir), accuracy (percent recovery was nearer to 100%), precision (percent relative standard deviation was less than 2.0%) and robustness (system suitability values are within limits). The stability indicating method was performed by under the various stress conditions. Degradants did not interfere with lamivudine and dolutegravir detection. The developed method can suggest that quantification of lamivudine and dolutegravir in quality control of analytical laboratories.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.