An FMEA-driven holistic approach to quality risk management for new product introduction in pharmaceutical commercial plant: navigating global regulatory landscapes
Abstract
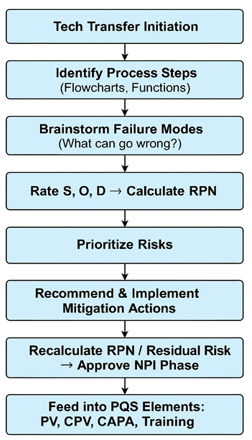
This review presents a comprehensive, regulatory-aligned strategy for conducting Quality Risk Assessments (QRAs) during New Product Introduction (NPI) in pharmaceutical commercial manufacturing. Focusing on the application of Failure Mode and Effects Analysis (FMEA), the paper offers a practical framework for identifying, evaluating, and mitigating risks across the NPI lifecycle. Leveraging international regulatory guidelines—such as ICH Q9, WHO TRS, PIC/S Annex 20, USFDA, EMA, MHRA, ANVISA, SAHPRA (formerly MCC), and ASEAN GMP standards—the study outlines a systematic and proactive approach to Quality Risk Management (QRM). The FMEA methodology is examined in detail, emphasizing its role in risk prioritization using Risk Priority Numbers (RPNs), integration with the Pharmaceutical Quality System (PQS), and alignment with regulatory expectations for validation, cleaning, documentation, and supply chain readiness. Key failure modes, causes, and mitigations related to process scale-up, analytical method transfer, raw material variability, cleaning validation, packaging integrity, and transport logistics are outlined. Additionally, a structured FMEA template is introduced, supporting consistent, objective risk evaluations across multidisciplinary teams. This FMEA-driven model enhances decision-making, ensures compliance, and improves patient safety during the transition from development to commercial scale.
Full text article
Generated from XML file
Authors
Vyawhare , N. A. . (2025). An FMEA-driven holistic approach to quality risk management for new product introduction in pharmaceutical commercial plant: navigating global regulatory landscapes. International Journal of Research in Pharmaceutical Sciences, 16(3), 24–45. https://doi.org/10.26452/ijrps.v16i3.4788
Copyright (c) 2025 International Journal of Research in Pharmaceutical Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.