Abstract
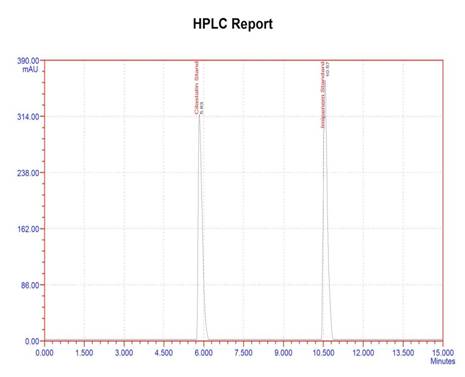
A combined dosage form of cilastatin and imipenem has been simultaneously determined by adopting an HPLC method. Stability indicating studies have been performed under various stress conditions. The reported method adopts Inertsil ODS C18 (250 mm x 4.6 mm, 5μm) column as stationary phase and a mobile phase consisting of methanol: acetonitrile: acetate buffer in the ratio of 70:25:05(v/v) at a pH 5.2 and an UV detector at 217 nm. Linear calibration curves for proposed method are arrived in the concentration ranges of 1-6 µg/mL for both cilastatin and imipenem (R2 > 0.999 for both drugs). The method is validated in terms of precision, ruggedness, robustness and accuracy. The developed method has been applied successfully to analyse marketed pharmaceutical formulation and vial samples. Degradation studies have been carried out with cilastatin and imipenem by exposing the drugs to various stress conditions like photolytic, aqueous acid, base, thermal and peroxide conditions. The proposed method successfully separated the drug from its degradation products. Hence, it can be employed as stability-indicating method for the determination of cilastatin and imipenem drugs in pure and formulations.
Full text article
Generated from XML file
Authors
Masimukku Siva Kishore, & Chintala Rambabu. (2023). Stability-indicating HPLC method development and validation for simultaneous determination of cilastatin and imipenem in pharmaceutical dosage forms. International Journal of Research in Pharmaceutical Sciences, 8(4), 560–567. Retrieved from https://ijrps.com/home/article/view/4620
Copyright (c) 2017 International Journal of Research in Pharmaceutical Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.