Abstract
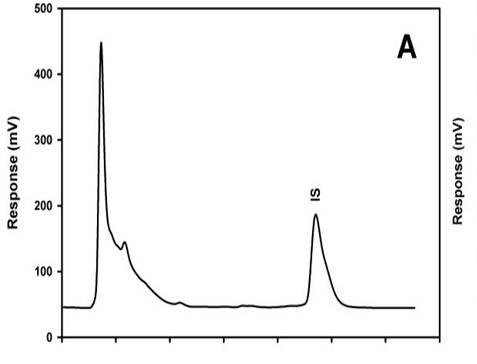
A simple and rapid method with HPLC-UV detection was developed for estimation of ibuprofen using only 50 µl of plasma samples. Analyses were performed on a Symmetry C18 column and UV detection at 196 nm. The method was validated according international criteria and it was used for quantification of ibuprofen plasma levels after p.o. administration of 17.8 mg/kg of the drug alone and combined with 17.8 mg/kg of caffeine to healthy male Wistar rats. Retention times for ibuprofen and mefenamic acid were < 5 min. No caffeine interference was found. Linearity was assessed with plasma solutions of 2.5‒100 µg/ml. R2 value was more than 0.999 (p < 0.05). The intra-day and inter-day precision, expressed as relative standard deviation, and accuracy, expressed as relative error, were < 15%. Stability was proved for four weeks at −20 °C. After concomitant caffeine administration no significant differences in ibuprofen plasma levels were found (p>0.05). The analytical assay is reliable and sufficiently sensitive for single-dose pharmacokinetic studies utilizing a small plasma sample. A large number of samples can be processed and run in a relatively short period of time.
Full text article
Generated from XML file
Authors
José Raúl Medina, Helgi Jung, Marcela Hurtado, Olivia Soria, & Francisco Javier López-Muñoz. (2023). Simple and rapid determination of ibuprofen without caffeine interference by HPLC-UV detection: application to pharmacokinetic studies in rats. International Journal of Research in Pharmaceutical Sciences, 8(1), 1–5. Retrieved from https://ijrps.com/home/article/view/4526
Copyright (c) 2018 International Journal of Research in Pharmaceutical Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.