Abstract
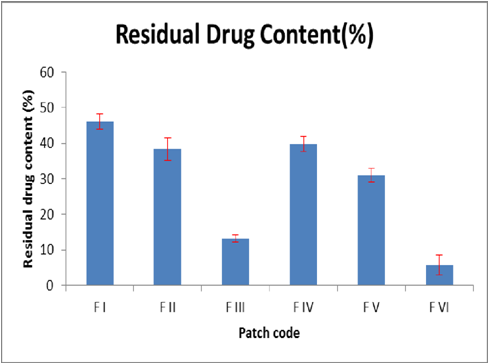
The study was intended to surpass the related problems of reduced oral bioavailability, to reduce the serious side effects associated with other routes and to study the suitability of formulating the drug into a mucoadhesive buccal patch for the systemic drug delivery. The mucoadhesive buccal film of imipramine hydrochloride was prepared using varying concentrations of HPMC E15 and Carbopol 940 by Solvent casting technique, which produced a transparent colourless patch. Physical evaluation, in-vitro drug release studies, ex-vivo drug permeation studies, histopathological studies, stability studies etc. were carried out on these formulated buccal patches and responses such as in-vitro drug release and ex-vivo drug permeation being used for optimization. The average thickness of the patch was found to be in the range of 0.01746 to 0.2488µm. The in-vitro drug release studies showed 93.39% drug release from formulation FVI and the stability studies carried out at two different temperatures showed patches remained stable even after 45 days. This study concludes that mucoadhesive buccal patches that were prepared were safe, convenient and effective formulation for the systemic delivery of an antidepressant drug, imipramine hydrochloride. Besides this, it provides sustained delivery of the drug into the systemic circulation and has least gastrointestinal side effects compared to the marketed formulation.
Full text article
Generated from XML file
Authors
Chinmayi Ramachandran, Sivapriya G Nair, Gopika S Kumar, & Vidya Viswanad. (2023). Formulation and evaluation of mucoadhesive buccal patch containing Imipramine hydrochloride. International Journal of Research in Pharmaceutical Sciences, 9(4), 1289–1302. Retrieved from https://ijrps.com/home/article/view/4458
Copyright (c) 2018 International Journal of Research in Pharmaceutical Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.