Abstract
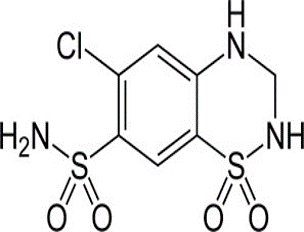
A simple and rapid UPLC-MS/MS method has been developed and validated for the analysis of Olmesartan medoxomil, Hydrochlorothiazide, Amlodipine besylate and Telmisartan as internal standard in plasma. Sample was pre- pared in LLE. LC separation was achieved in Acquity TQD. TQD detector and analytical column of RP-18 (50 mm* 2.1mm, 1.7 micron) at 40°C. The mobile phase consisted Acetonitrile and Ammonium acetate as a gradient elution up to 0.8 minutes/42% A, 5/10%, 6/10%, 6.1/42% and 10/42%. Total run time was 10 minutes operating with flow rate 0.3mL/ minutes. Mass spectroscopy detection was performed by negative and positive ion mode electro spray Olmesartan medoxomil, Hydrochlorothiazide and Amlodipine besylate, Telmisartan respectively. The method proved to be specific and linear over the range (40.34 to 8092.75) ng/mL for Olmesartan Medoxomil, Hydrochlorothiazide and Amlodipine besylate. This technique also showed high sensitivity with a 2.14, 1.86 and 1.12 ng LOD and 6.28, 4.21 and 3.86 ng LOQ Olmesartan Medoxomil, Hydrochlorothiazide and Amlodipine besylate respectively. Percentage recovery ranged from 86 to 103% for Olmesartan medoxomil, Hydrochlorothiazide, Amlodipine Besylate and from 58 to 110 for Telmisartan. CV of inter and intra precision was found within 15%. The LC–MS/MS assay reported in this paper is rapid, simple, specific and sensitive for simultaneous quantification of Olmesartan Medoxomil, Hydrochlorothiazide and Amlodipine Besylate in human plasma and is fully validated according to commonly acceptable FDA guidelines. And the method can be useful for BA/BE studies and routine therapeutic drug monitoring with the desired precision and accuracy.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.