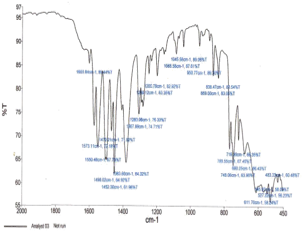
Abstract
Diclofenac sodium is a common and widely-used drug for the treatment of pain, inflammation and also migraine. Unfortunately, it undergoes extensive first-pass hepatic metabolism when administered through the oral systemic route. Thus, this study will be about the formulation of mucoadhesive buccal tablets of diclofenac sodium that can prevent the extensive metabolism of the drug, which in turn increases its bioavailability inside the systemic circulation. This formulation might also reduce the dosing frequency, which can lead to a better patient compliance to the medication. The formulations would be using natural polymers such as acacia gum and chitosan as the mucoadhesive polymers. Ethylcellulose (EC) was included as the backing layer of the buccal tablets, along with other excipients. In total, four different formulations were prepared with the varying concentration of the natural polymers. The formulated buccal tablets have been evaluated for their general appearance, thickness, hardness, weight variation, friability, ex-vivo mucoadhesion time and other in-vitro tests such as swelling and dissolution studies. The finding of this study confirmed that Formulation 3 (F3) had the best properties of mucoadhesive buccal tablets as it displayed the highest in-vitro swelling index and in-vitro dissolution profile, also with the longest ex-vivo mucoadhesion time.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.