Abstract
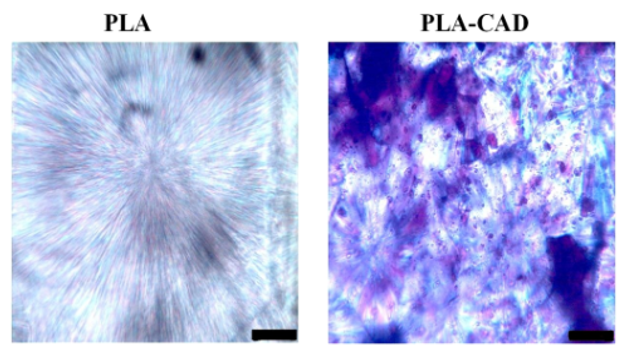
Wounds have been occurring as long as the existence of life. Presently available advanced wound care products for dressing are beyond the reach of the majority Indian population, and they also do not completely fulfil the required benefits of therapeutic value. The dermal patch technology is the best-known and widely used approach for delivering drugs. It has been proven to be the fastest, easiest, safest and most economical way for drug delivery. The use of biodegradable polymers in wound management has been brought into prominence with new innovations in drug delivery system. Thus with a new dimension for the use of polymeric materials in or as wound healing, drug delivery devices involves incorporation of biodegradability into the drug delivery system. A number of degradable polymers are potentially useful for drug delivery including a variety of synthetic and natural substances such as Poly Lactic acid, Poly Crypolactone, Chitosen etc. Among all these Poly (lactic acid) (PLA) is the most readily biodegradable polymer in the surgical field. The biodegradable polymers have gained growing importance in the medical area, and these have been used in a wide number of applications in the human body, such as surgical sutures, controlled drug release systems, artificial skins, guides for nerves, veins and artificial arteries and orthopaedic devices. Biodegradable polymers have several physical and chemical characteristics, such as molecular mass average and distribution, glass transition and/or melting temperatures, monomer ratios and sequencing for copolymers. The knowledge of physicochemical characteristics of Poly Lactic acid polymers essential to understand its thermo-mechanical performance. In order to achieve appropriate wound healing, sustained release of the drug from the bio-degradable patch is necessary. So the assessment of the interaction between the drug and polymer is indispensable.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.