Abstract
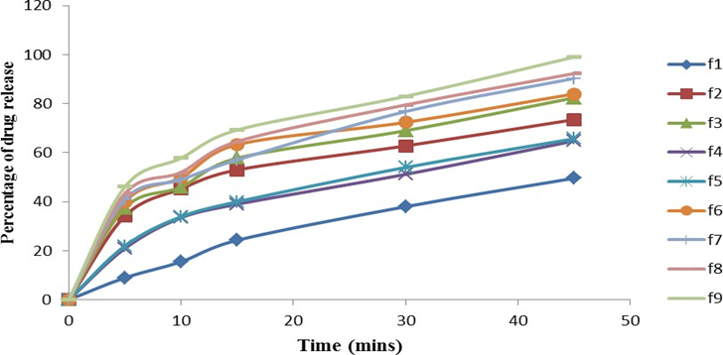
The present investigation involves formulation and assessment of Nevirapine immediate release tablets with a view to release the drug immediately to maintain the consistency into the plasma. Nevirapine is an anti -HIV drug belongs to BCS class-II in order to increase the solubility and dissolution rate of Nevirapine, the solid dispersion technique was employed by using the carriers like HPMC, Xanthan gum, Starch and sodium starch glycollate at different concentrations. Solid dispersions were prepared by solvent evaporation and surface adsorption method. The prepared solid dispersions were blended with microcrystalline cellulose, Cross carmellose sodium, Magnesium stearate and talc, then compressed into tablets. The formulated tablets complied for all official tests for the tablets. In vitro dissolution studies were carried out for all formulations using 0.1N HCl for 45 minutes at 37±0.5 oC. In vitro dissolution studies showed that F9 formulation containing sodium starch glycollate (1%) showed improved dissolution rate of 99.05% of drug release within 45 minutes compared to pure drug.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.