Abstract
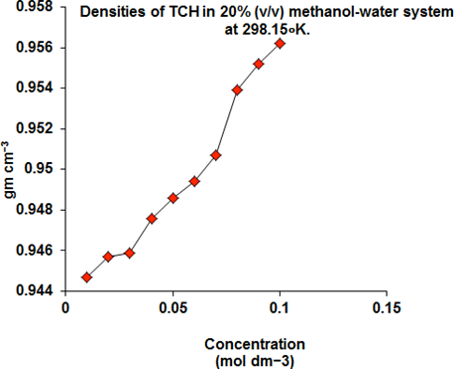
In this research article, we have described to Viscometric properties of Tetracyclin hydrochloride (TCH) are measured in 20% (v/v) methanol-water system 298.15∘K. The related parameters are the experimental values of viscosity (????) allow to determine relative viscosity (????????), viscosity ????-coefficient of the Jones-Dole equation, free energies of activation of viscous flow Δ????10≠ and Δ????20≠ per mole solvent and solute respectively. The excess molar volume, excess viscosity, excess Gibb’s free energy, and interaction parameter of Grunberg and Nissan have also been calculated. These studies help in characterizing the structure and properties of solutions. The addition of an organic solvent to water brings about a sharp change in the solvation of ions. The dependence of these properties with temperature are shown. The results are interpreted in terms of solute-solvent interaction.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.